Periodic Table of Elements - Elements Database
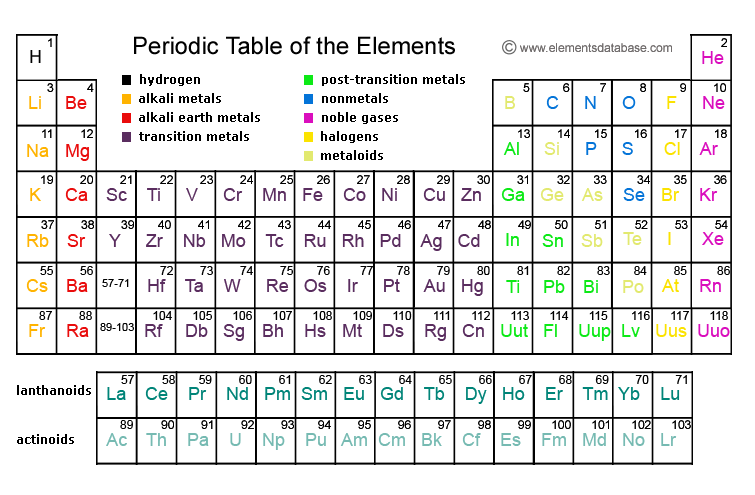
Periodic TableOur periodic table of chemical elements presents complete information on the chemical elements including the chemical element symbol, atomic number, atomic weight and description. The links below lead to the detailed description of most well known chemical elements. Our periodic table information can be useful for chemistry and physics students, as well as science researchers.You can test your Periodic Table knowledge with our Periodic Table Quiz.
Dynamic Periodic Table
|
Actinium - Ac Aluminum - Al Americium - Am Antimony - Sb Argon - Ar Arsenic - As Astatine - At Barium - Ba Berkelium - Bk Beryllium - Be Bismuth - Bi Bohrium - Bh Boron - B Bromine - Br Cadmium - Cd Caesium - Cs Calcium - Ca Californium - Cf |
Carbon - C Cerium - Ce Chlorine - Cl Chromium - Cr Cobalt - Co Copernicium - Cn Copper - Cu Curium - Cm Darmstadtium - Ds Dubnium - Db Dysprosium - Dy Einsteinium - Es Erbium - Er Europium - Eu Fermium - Fm Flerovium - Fl Fluorine - F Francium - Fr |
Gadolinium - Gd Gallium - Ga Germanium - Ge Gold - Au Hafnium - Hf Hassium - Hs Helium - He Holmium - Ho Hydrogen - H Indium - In Iodine - I Iridium - Ir Iron - Fe Krypton - Kr Lanthanum - La Lawrencium - Lr Lead - Pb Lithium - Li |
Livermorium - Lv Lutetium - Lu Magnesium - Mg Manganese - Mn Meitnerium - Mt Mendelevium - Md Mercury - Hg Molybdenum - Mo Neodymium - Nd Neon - Ne Neptunium - Np Nickel - Ni Niobium - Nb Nitrogen - N Nobelium - No Osmium - Os Oxygen - O Palladium - Pd |
Phosphorus - P Platinum - Pt Plutonium - Pu Polonium - Po Potassium - K Praseodymium - Pr Promethium - Pm Protactinium - Pa Radium - Ra Radon - Rn Rhenium - Re Rhodium - Rh Roentgenium - Rg Rubidium - Rb Ruthenium - Ru Rutherfordium - Rf Samarium - Sm Scandium - Sc |
Seaborgium - Sg Selenium - Se Silicon - Si Silver - Ag Sodium - Na Strontium - Sr Sulphur - S Tantalum - Ta Technetium - Tc Tellurium - Te Terbium - Tb Thallium - Tl Thorium - Th Thulium - Tm Tin - Sn Titanium - Ti Tungsten - W Ununoctium - Uuo |
Ununpentium - Uup Ununseptium - Uus Ununtrium - Uut Uranium - U Vanadium - V Xenon - Xe Ytterbium - Yb Yttrium - Y Zinc - Zn Zirconium - Zr |
Printable Periodic Table | Black & White Printable Periodic Table
The periodic table images below are copyrighted by ElementsDatabase.com and may not be used without permission.
Origins of the Periodic Table of Elements
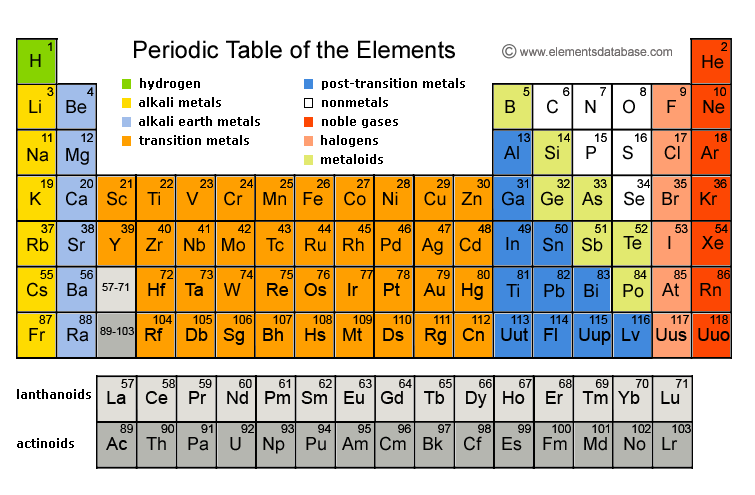
The Periodic Table displays all known chemical elements which are grouped by chemical properties and atomic structure.
Copper, silver, gold, mercury, tin, lead, and other elements have been known since ancient times and were used to make jewelry, coins, and tools. Phosphorus became the first element to be discovered by Hennig Brand in 1649. It is known as the first scientific discovery of a chemical element.
Early Systematization Attempts
A total of 63 elements have been discovered by 1869. However, the first attempts at systematization occurred in 1829 and 1862. Johan Dobereiner grouped chemical elements into triads, and De Chancourtois formulated a chart with closely related elements.
Mendeleev's Periodic Table
It was only in 1869 when Dmitri Mendeleev, an inventor and chemist of Russian origin, discovered the Periodic Law and organized all chemical elements in columns and rows. The elements were organized based on their physical and chemical properties.
The Extended Periodic Table
It is still to be discovered how far the Periodic Table of Elements extends. According to the American scientist Glenn Seaborg, element 130 is the highest possible. Attempts were made to synthesize several new elements, including unbiseptium, unbihexium, unbiquadium, and unbibium.
Periodic Table Grouping
The Periodic Table includes 18 groups, and each group contains elements with similar chemical and physical properties of the outermost electron shells. The so called typical elements are found in the first two rows.
Groups
Group 1 of the Periodic Table groups together the alkali metals while group 2 contains all alkaline earth metals. The noble gases and halogens are in groups 18 and 17, respectively. The Periodic Table groups elements into the cobalt, chromium, vanadium, scandium, copper, cobalt, and other groups.
Periods
There are 7 periods of elements that group elements with similar properties. Period 1 contains two elements, helium and hydrogen while period 7 contains radioactive elements. The rare earth elements are found in period 6. Many period 6 elements are toxic, heavy, and radioactive.
Blocks
Blocks combine adjacent groups and are also called element families. There are 4 blocks in the Periodic Table - f, d, p, and s. The f block includes inner transition elements and the d block is made of transition elements. The p block includes post-transition metals, semimetals, and nonmetals, with the exception of helium and hydrogen. The s block contains alkaline earths and alkali metals.
Major Categories
The major categories are metalloids, nonmetals, and metals, and most elements in the Periodic Table are metals. Metals are malleable, shiny, and ductile while nonmetals lack metallic properties and are volatile. Metalloids share properties with both nonmetals and metals.
Periodic Table Curious Facts
The Most Expensive Element
Lutetium is a metal and the most expensive chemical element available. The price of 1 gram is $100. Francium, however, is the most expensive element that can be produced. A small amount will cost a few billion.
The Lightest and Heaviest Element
Hydrogen is the lightest element, and it is also the most abundant one. Hydrogen has important commercial applications, for example, hydrogen fuel cells and the manufacture of chemical products. Uranium is the heaviest element that occurs freely in nature. Ununoctium is heavier and the heaviest known chemical element, but it is manmade.
The Rarest Element
The rarest element is astatine, and scientists estimate that the total amount found is less than 1 gram. CERN researchers suggest that its isotopes can be used in cancer treatment therapies.
Precious Metals
The group of precious metals includes elements such as palladium, ruthenium, platinum, iridium, and gold. Other precious metals include osmium, ruthenium, silver, and rhodium. They are used for coinage, jewelry, and alloys and have different commercial applications.
Artificially Made Elements
Some elements are not found in nature but are synthesized in laboratory settings. They are also called synthetic elements and have radioactive properties. Fermium and einsteinium are the first artificially made elements which were discovered in 1952. Today, there are 20 known synthetic or artificially made elements, among which copernicium, roentgenium, dubnium, ununseptium, lawrencium, and others.
The Most Abundant Element in the Universe
About 75 percent of the mass of the universe consists of hydrogen, making it the most abundant element. It is a colorless and odorless gas that makes up nuclear matter. The second most abundant is helium, which accounts for about 25 percent of the mass. Other elements include oxygen, iron, neon, magnesium, carbon, and nitrogen.
How Many Radioactive Elements?
There are 37 radioactive elements in the Periodic Table. Many radioactive isotopes have been isolated as well. The list of radioactive elements includes berkelium, radon, polonium, californium, thorium, and others.
What is the Most Radioactive Element?
Polonium, which is classified as a metal and metalloid, is the most radioactive element that has no stable isotopes. It occurs naturally in very low concentrations. Lawrencium and nobelium are also highly radioactive elements.
Most dense? Least dense?
Osmium is the densest known chemical element with density of 22.61 g/cm3. Other elements with high density include platinum, rhenium, gold, thallium, berkelium, and americium. The least dense element is hydrogen while lithium is the least dense solid metal.
Chemistry doesn't have to be boring. Learn more about the The Chemistry of Teeth Whitening. If you are wondering what does have chemistry to do with money then read The Chemistry of Paper Money, The Chemistry of Polymer Banknotes and The Chemistry of Credit Cards. Learn why precious metals are so special. Learn more about banking, money and financial matters at Canadian Banks. If you wondered what does glycemic index mean, what are carbohydrates (simple carbs and complex carbs), how does a low carb diet work, and can you drink sugar alcohols you have come to the right place :).